Bormioli pharma
Innovating
for a better life
Bormioli Pharma is part of the Gerresheimer Group, a global provider of systems and solutions for the pharma and biotech industry. For the full story, latest updates, and everything about the company, simply head to Gerresheimer’s website.
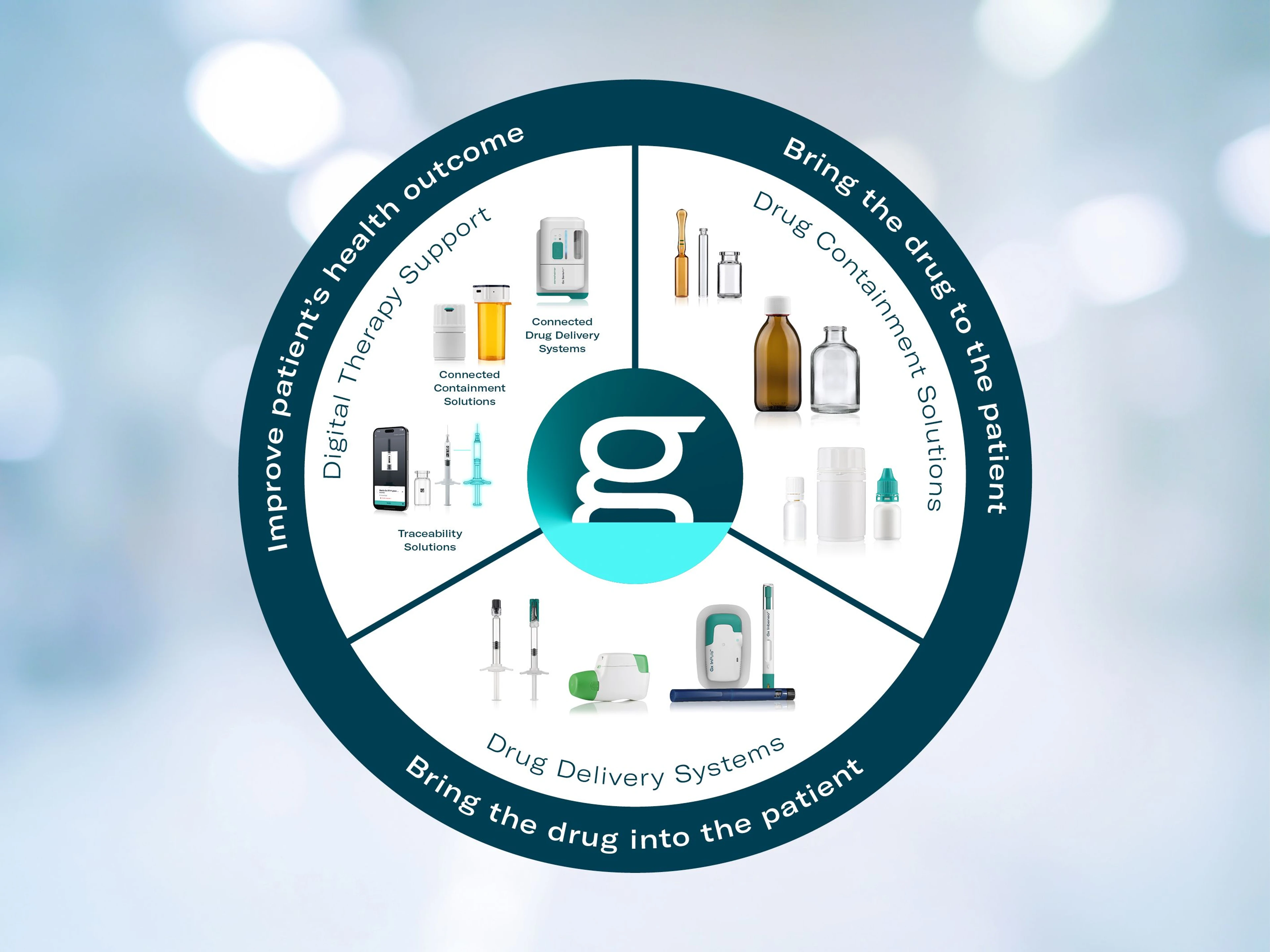
Our Complete Offer
Bormioli Pharma’s extensive product range includes pharmaceutical primary packaging as well as systems and solutions for the safe and convenient administration of medicines. Integrated into Gerresheimer’s portfolio, Bormioli Pharma products contribute to the most complete and high-quality offering in the industry.